Now Enrolling: LEGION-100 Phase 2 Trial for Metastatic Castration-Resistant Prostate Cancer. Find A Location Near You ›
SYNC-T™
Solid Tumor
Immunotherapy Platform
Novel personalized combination in situ immunotherapy platform designed to synchronize the location of 3 key components necessary to educate the immune system to recognize and attack cancer: patient-specific tumor antigens, our proprietary multi-target biologic drug, and immune cells
Cancer by the Numbers
Estimated number of people in the U.S. living with metastatic lung, breast, and prostate cancer
Syncromune is currently focused on developing therapies for metastatic non-small cell lung cancer, metastatic breast cancer, and metastatic castration-resistant prostate cancer. There is a large unmet need, as currently most metastatic solid tumor cancers are incurable.

Metastatic NSC Lung Cancer
0

Metastatic Breast Cancer
0

Metastatic Prostate Cancer
0
Source: DelveInsight. Market Insight, Epidemiology, and Market Forecast – 2032.. mCRPC & mBC prevalence, US mNSCLC annual deaths, OUS mNSCLC incidence. Published September 2023.
The Challenge
Cancer has many mechanisms and ways to avoid, overwhelm, and suppress the immune system. If only one or two of those mechanisms are targeted by a drug or therapy, cancer can compensate and use other means to overcome the immune system. Currently, responses to immunotherapy vary by cancer type, and on average, only 15% to 30% of patients with solid tumors respond to single-target IV immunotherapy1, largely because of the multi-faceted immunosuppressive abilities of cancer.
Ideally, combining two or more drugs could result in improved patient outcomes, but the combination of systemic IV immunotherapies thus far in metastatic castration-resistant prostate cancer (mCRPC) trials and other treatment-resistant cancers have demonstrated limited benefit with high toxicities, adverse events, and discontinuation rates due to lack of tolerability 2,3.
Given the complex and multifactorial nature of the mechanisms mediating tumor immune evasion in mCRPC and other metastatic solid tumor cancers, it is becoming increasingly clear that new cancer immunotherapies need to implement a combination of numerous therapeutic agents that elicit multiple immuno-pharmacologic effects while also minimizing systemic autoimmune side effects.
A Bold New Approach to Treating Metastatic Cancer
Thinking outside the box to fight incurable cancers
SYNC-T™: A Novel Combination Multi-Target Approach
SYNC-T Therapy is a combination in situ immunotherapy platform designed to synchronize the location of 3 key components necessary to educate the immune system to recognize and attack cancer: patient-specific tumor antigens, our multi-target biologic drug, and immune cells.
The SYNC-T Therapy Platform is Designed to:
- Provide personalized in situ cancer therapy through partial oncolysis of a patient’s tumor to release the patient's own tumor antigens and enhance immune activation
- Utilize a combination approach of patient-specific tumor antigen release and multi-target drug infusion to both reduce immune suppression and enhance immune activation
- Synchronize the location of 3 key components (patient-specific tumor antigens, the multi-target drug, an immune cells) necessary to educate the immune system to recognize and attack cancer
- Minimize systemic exposure and side effects by infusing the low dose, high concentration multi-target drug directly into the tumor
Play Video
SYNC-T Animation: How it Works
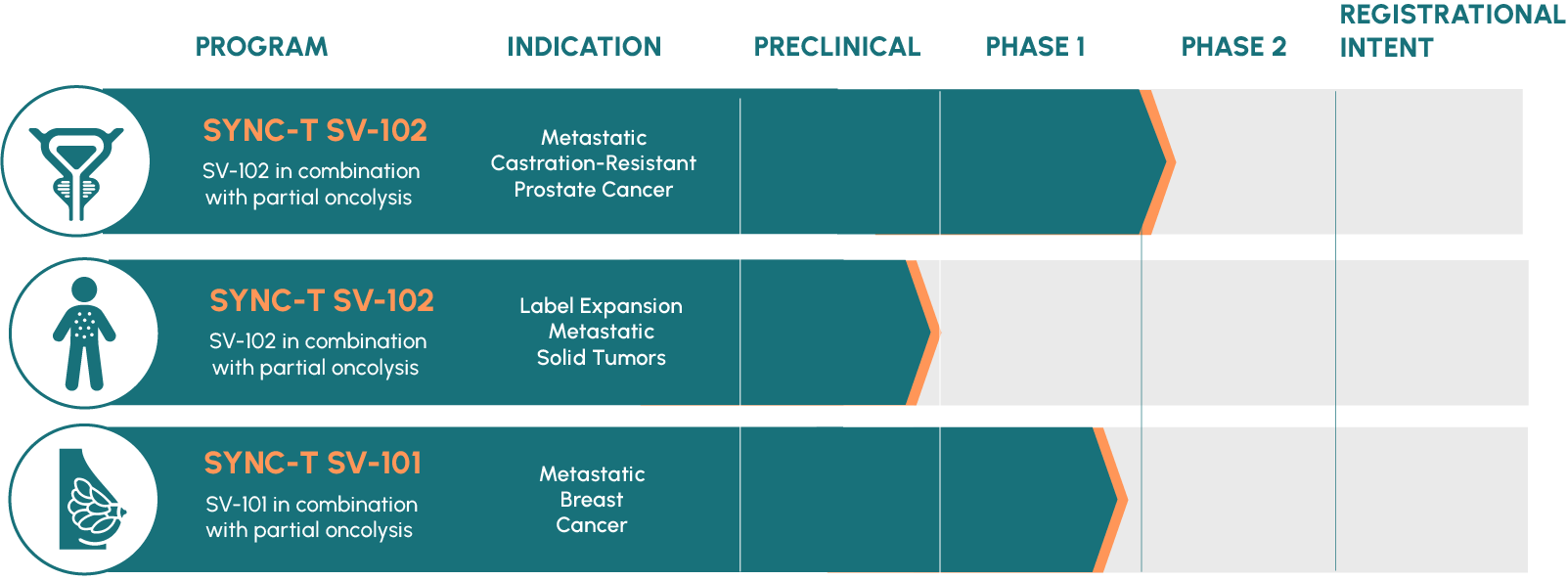
Pipeline
Our current pipeline focuses on developing the SYNC-T platform therapy for metastatic solid tumor cancers. You can learn more about SYNC-T trials HERE.
Key Scientific Presentations & Publications
Syncromune is committed to advancing science and developing cancer therapies to leverage the full potential of combination multi-target approaches. We strive to publish our trials as well as investigator-initiated work in peer-reviewed journals and present our data at medical congresses to contribute to furthering cancer research.
Prostate Cancer Foundation 2025 Scientific Retreat | October 23, 2025 | Poster Presentation
Link, C.J., Hobbs, E.P. (2025, October 23-25). [Poster presentation]. Clinical Activity and Safety of SYNC-T Therapy SV-102 in Patients with Metastatic Prostate Cancer. Prostate Cancer Foundation (PCF) Scientific Retreat 2025, Carlsbad, CA, United States.
American Society of Clinical Oncology (ASCO) 2025 Annual Meeting | May 31, 2025 | Presentation
Tong, R.T. (2025, May 30-June 3). Clinical responses to SYNC-T Therapy: In situ personalized cancer vaccination with intratumoral immunotherapy in patients with metastatic castration-resistant prostate cancer (mCRPC). In Sarah Nikiforow, MD, PhD and Meredith Pelster, MD (Chairs), Developmental Therapeutics – Immunotherapy [Conference presentation]. ASCO 2025, Chicago, IL, United States.
American Association for Cancer Research (AACR) 2024 Annual Meeting | April 7, 2024 | Presentation
American Association for Cancer Research (AACR) 2024 Annual Meeting | April 5-10, 2024 | Poster Abstract
American Society of Clinical Oncology (ASCO) 2025 Annual Meeting | May 31, 2025 | Presentation
Tong, R.T. (2025, May 30-June 3). Clinical responses to SYNC-T Therapy: In situ personalized cancer vaccination with intratumoral immunotherapy in patients with metastatic castration-resistant prostate cancer (mCRPC). In Sarah Nikiforow, MD, PhD and Meredith Pelster, MD (Chairs), Developmental Therapeutics – Immunotherapy [Conference presentation]. ASCO 2025, Chicago, IL, United States.
American Association for Cancer Research (AACR) 2024 Annual Meeting | April 7, 2024 | Presentation
Link, C.J. (2024, April 5-10). Systemic responses to SYNC-T therapy: in situ personalized cancer vaccination with intratumoral infusion of multi-target immunotherapy in patients with metastatic castrate-resistant prostate cancer (mCRPC). In Ecaterina Dubrava & Ulka N. Vaishampayan (Chairs), Cancer Vaccines: Ready for Prime Time? [Conference presentation]. AACR 2024, San Diego, CA, United States.
American Association for Cancer Research (AACR) 2024 Annual Meeting | April 5-10, 2024 | Poster Abstract
James B. DuHadaway, Alexander J. Muller, Lisa D. Laury-Kleintop, U. Margaretha Wallon, Susan K. Gilmour, Marie Webster, Jason R. Williams, Gabriela R. Rossi, Mario R. Mautino, Jonathan Lewis, Charles J. Link, George C. Prendergast. Cryo-immune vaccination (CIV) by SYNC-T therapy: Preclinical modeling of a novel device-multidrug immunotherapeutic approach to eradicate advanced metastatic cancers [poster abstract]. In: Proceedings of the American Association for Cancer Research Annual Meeting 2024; Part 2 (Late-Breaking, Clinical Trial, and Invited Abstracts); 2024 Apr 5-10; San Diego, CA. Philadelphia (PA): AACR; Cancer Res 2024;84(7_Suppl):Abstract nr LB355.
REFERENCES
- Das S, Johnson DB Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. Journal for ImmunoTherapy of Cancer 2019;7:306. doi: 10.1186/s40425-019-0805-8
- Sharma P, Pachynski RK, Narayan V, Fléchon A, Gravis G, Galsky MD, Mahammedi H, Patnaik A, Subudhi SK, Ciprotti M, Simsek B, Saci A, Hu Y, Han GC, Fizazi K. Nivolumab Plus Ipilimumab for Metastatic Castration-Resistant Prostate Cancer: Preliminary Analysis of Patients in the CheckMate 650 Trial. Cancer Cell. 2020 Oct 12;38(4):489-499.e3. doi: 10.1016/j.ccell.2020.08.007. Epub 2020 Sep 10. PMID: 32916128.
- Shenderov E, Boudadi K, Fu W, et al. Nivolumab plus ipilimumab, with or without enzalutamide, in AR-V7-expressing metastatic castration-resistant prostate cancer: A phase-2 nonrandomized clinical trial. Prostate. 2021 May;81(6):326-338. doi: 10.1002/pros.24110. Epub 2021 Feb 26. PMID: 33636027; PMCID: PMC8018565.
On this page you may select links to key publications from peer-reviewed journals as well as to materials presented at scientific conferences relating to Syncromune’s research and development of its product candidates. Investigational products and their uses mentioned on this website have not been determined safe and effective by the relevant regulatory authorities, including the US Food and Drug Administration or other applicable agencies in other countries. The content of each work is the property of the respective copyright holder and may not be further distributed without their express permission. The information in these materials may not be current or comprehensive, and Syncromune undertakes no obligation to correct or update such information.